 Transcutaneous transmission leaves skin intact
Transcutaneous transmission leaves skin intact No acoustic feedback
No acoustic feedback No permanent wound, low risk for skin infections
No permanent wound, low risk for skin infections Natural sound and directional hearing
Natural sound and directional hearing Shallow, flexible implant placement in the temporal bone
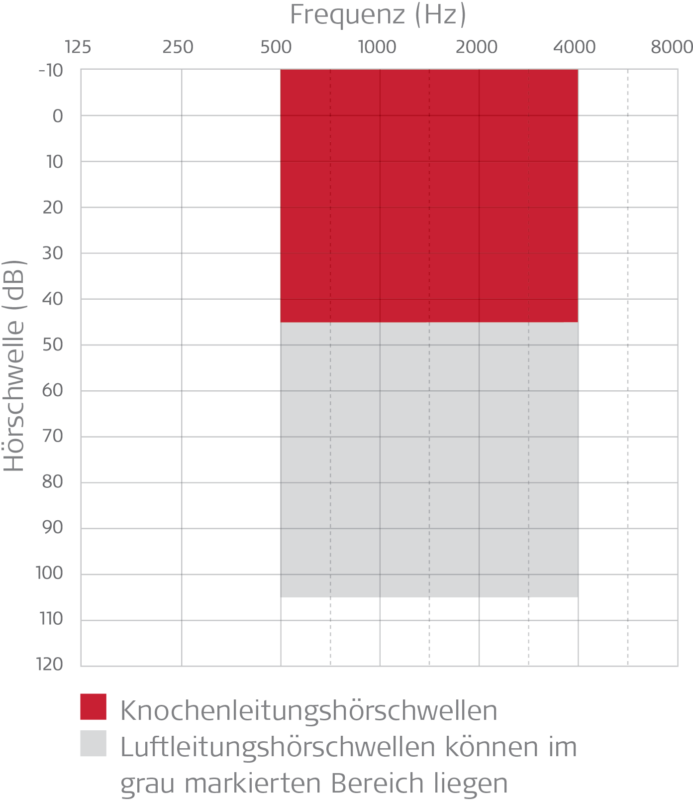
Shallow, flexible implant placement in the temporal bone Stable audiological results independent of outer or middle ear condition
Stable audiological results independent of outer or middle ear condition Free ear canal leads to better ventilation and mimized infection risk
Free ear canal leads to better ventilation and mimized infection risk MRI conditional at 1.5 Tesla* without magnet removal**
MRI conditional at 1.5 Tesla* without magnet removal** Comfortable and discreet to wear without additional fixation
Comfortable and discreet to wear without additional fixation 8-10 days of battery life
8-10 days of battery life Easy to use
Easy to use Streaming options
Streaming options Splash-proof or waterproof with cover
Splash-proof or waterproof with cover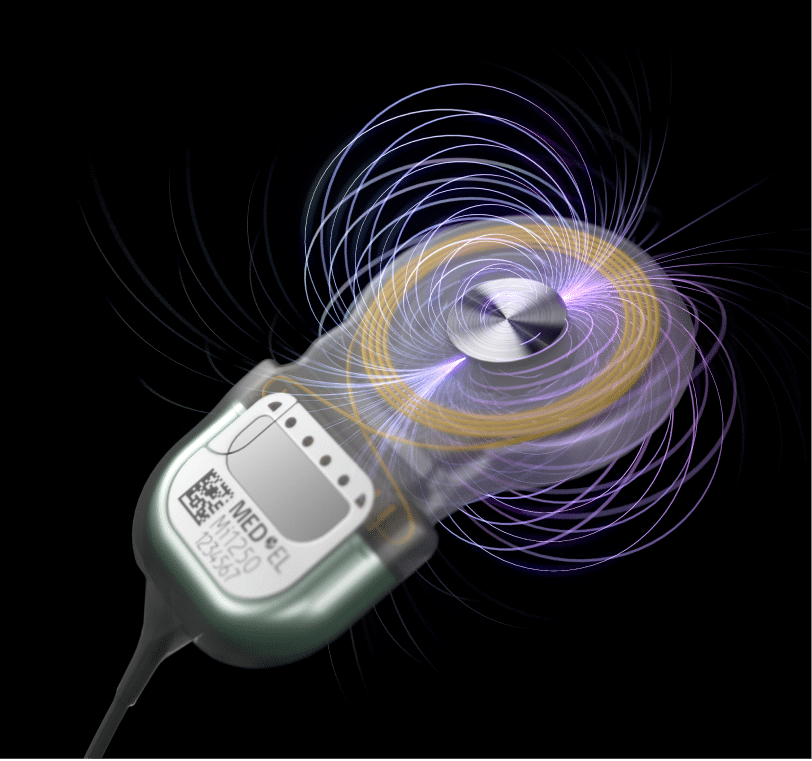
By subscribing to our newsletter, you stay up-to-date on the best practices in hearing implant technology.
hearbetter is an initiative of