BONEBRIDGE
Bone conduction implant
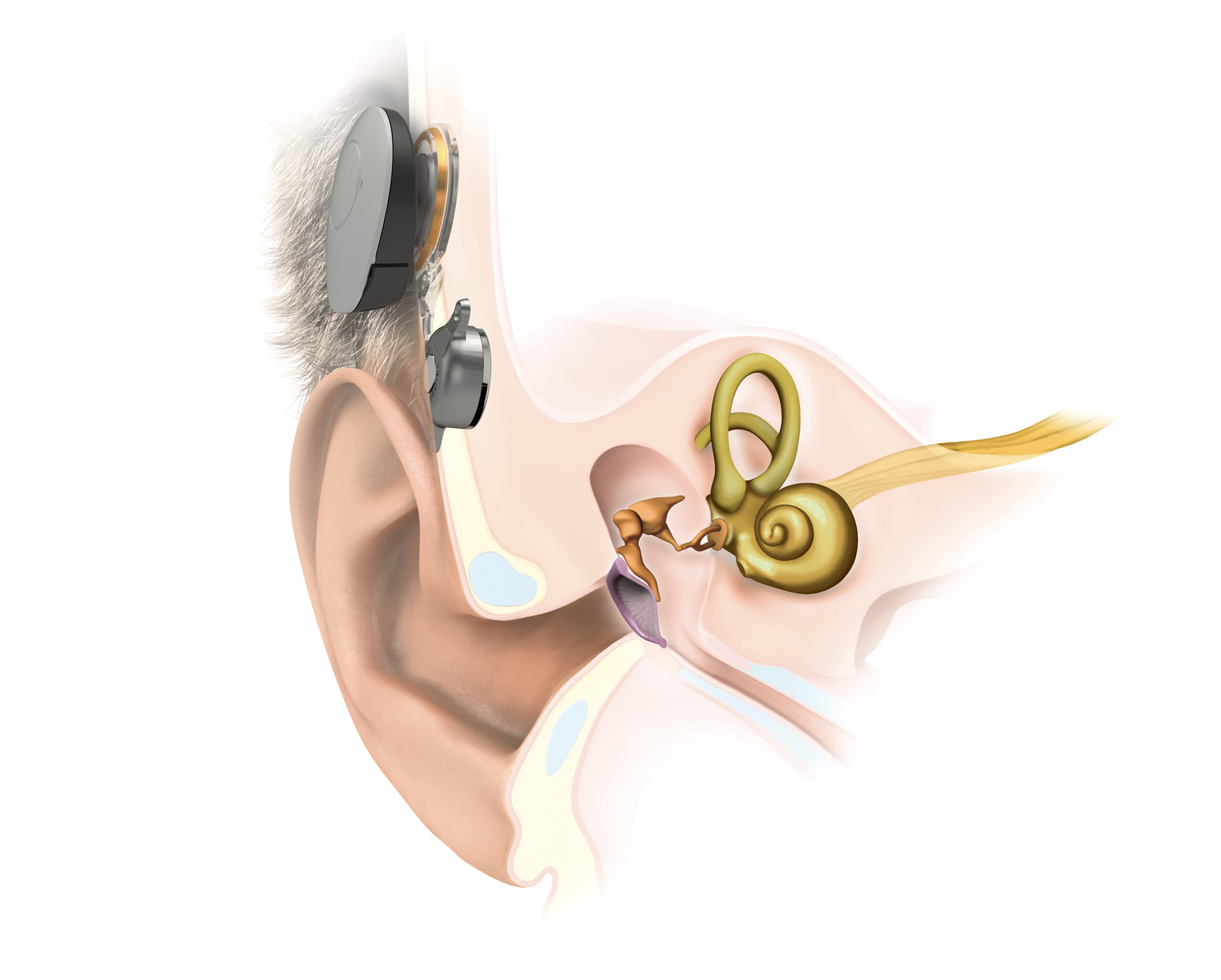
For conditions of the outer or middle ear with
conductive or mixed hearing loss
Key features of the BONEBRIDGE bone conduction implant system
- Transcutaneous transmission leaves skin intact
- No permanent wound, low risk for skin infections
- Shallow, flexible implant placement in the temporal bone
- Free ear canal leads to better ventilation and mimized infection risk
- No acoustic feedback
- Natural sound and directional hearing
- Stable audiological results independent of outer or middle ear condition
- MRI conditional at 1.5 Tesla* without magnet removal**
SAMBA 2 2 audio processor
- Comfortable and discreet to wear without additional fixation
- Easy to use
- 8-10 days of battery life
- Streaming options
- Splash-proof or waterproof with cover
*The BONEBRIDGE bone conduction implant ist MRI conditional. BONEBRIDGE users may be safely MRI scanned at 1.5 Tesla following the conditions detailed in the instructions for use.
** Unless required for diagnostic reasons.
The BONEBRIDGE bone conduction implant - for conditions of the outer or middle ear with conductive or mixed hearing loss
Indications:
- Conductive or mixed hearing loss
- Atresia
- Unilateral, bilateral
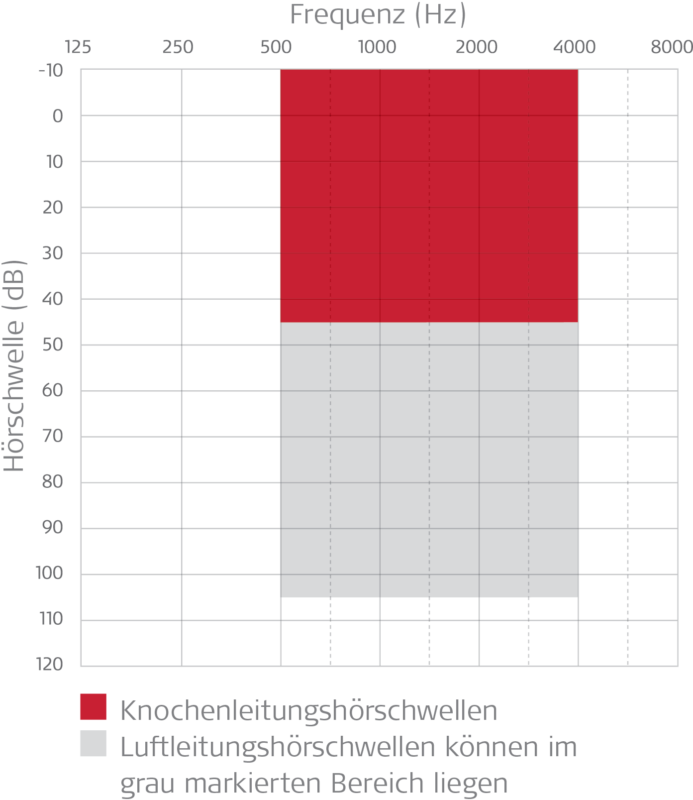
- Bone conduction thresholds: ≤ 45 dB at 500 - 4000 Hz - see red area in chart
- Anatomy allowing the placement of the implant
- No retrocochlear or central hearing disorders
- Realistic expectations
- Minimum age: 5 years
Is this hearing solution suitablefor your patients?
hearbetter helps you find the suitable hearing solution for your patients and connects you with experienced specialists.
Find a clinic for referral
Find a clinicShare experiences with the hearbetter community
Go to CommunityWhat users say about their BONEBRIDGE bone conduction implant
"INSERT USER TESTIMONIAL FROM YOUR AREA INSERT USER TESTIMONIAL FROM YOUR AREA INSERT USER TESTIMONIAL FROM YOUR AREA INSERT USER TESTIMONIAL FROM YOUR AREA"

User name
"INSERT USER TESTIMONIAL FROM YOUR AREA INSERT USER TESTIMONIAL FROM YOUR AREA INSERT USER TESTIMONIAL FROM YOUR AREA"

User name
"INSERT USER TESTIMONIAL FROM YOUR AREA INSERT USER TESTIMONIAL FROM YOUR AREA INSERT USER TESTIMONIAL FROM YOUR AREA"

User name
More information on MED-EL's BONEBRIDGE bone conduction implant and how it works: